
Confocal Microscopy and Live Cell Imaging

Microtubule-Dependent Regulation of HIF-1α
Hypoxia Inducible Factor-1 alpha (HIF-1α) is a transcription factor controlling angiogenesis that has been linked to breast, lung, prostate, and brain cancer cell survival. In fact, HIF-1α is overexpressed in more than 70% of human cancers and their metastases, as compared with the adjacent normal tissue. The upregulation of HIF-1α in many different tumors by many different mechanisms underscores the critical role HIF-1α plays in tumor biology. Rapidly growing tumor tissue can use up oxygen faster, creating hypoxic conditions. Increasing the blood supply is one of the ways to assure the cancer gets more nutrients. HIF’s ability to induce angiogenic activation is what makes it an important factor for tumor survival; therefore, HIF-1α appears to be a prime target for anticancer therapy.
Under normoxic conditions, HIF-1α is made in the cytoplasm and then rapidly degraded by the proteasome. In the presence of oxygen and iron, HIF-1α can be tagged for degradation by hydroxylation of its proline residues 402 and 564. This tagged molecule is recognized by the E3 ubiquitin ligase pVHL (von Hippel-Lindau tumor suppressor protein). In contrast, under hypoxia, ubiquitination does not occur because there is no hydroxylation of HIF-1α. In this case, unhydroxylated HIF-1α protein can proceed to the nucleus, where it heterodimerizes with HIFβ, then binds to HRE (HIF response elements). Once together, they can activate the transcription of over 40 genes that serve to promote cell survival under hypoxia, including the genes that promote angiogenesis, so that more oxygenated blood can reach hypoxic areas. Associated with increased cancer cell metastasis, tumor hypoxia can confer resistance to both chemotherapy and radiation, leading to poor patient survival. We would like to change the dismal clinical outcome facing some patients, by finding ways to eliminate the resistant and metastatic cancers. Inhibition of HIF-1α and discovery of new targets in the HIF-1 pathway represent attractive therapeutic options.
Studies performed in the Giannakakou laboratory have indicated that the relatively new small molecule drug 2ME2 has a dual anticancer effect, targeting both the microtubules (MT) involved in cell division; and HIF, involved in angiogenesis. Upon investigating the mechanism of action of 2ME2, we ultimately discovered that there is a broader mechanistic link between the disruption of the cytoskeleton and inhibition of angiogenesis. In fact, we found evidence that disruption of the microtubule cytoskeleton, HIF dysregulation, and the inhibition of angiogenesis are linked. We arrived at this mechanistic link by testing and comparing 2ME2 with other microtubule-targeting drugs that display antiangiogenic properties (Mabjeesh et al., Cancer Cell 2003). Due to their ability to affect not only cancer, but also endothelial cell MTs, the antiangiogenic properties of these drugs had not been previously correlated with the drugs’ effects on tumor cell MTs, but had been viewed solely as a product of inducing endothelial cell death. Our research sustains the concept that there is an important, common anti-angiogenic mechanism of action for the microtubule-targeting drugs.
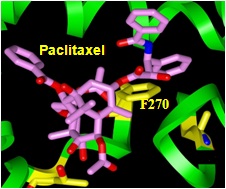
The Giannakakou laboratory found that 2ME2 downregulates HIF-1 at the post-transcriptional level, consequently inhibiting transcriptional activation of vascular endothelial growth factor (VEGF) expression (Mabjeesh et al., Cancer Cell 2003). This was shown in prostate and breast cancer cells. Moreover, using our taxol-resistant cells and drug-sensitive parental cells, we discovered that disruption of interphase microtubules is required for HIF-1α downregulation to occur (Mabjeesh et al., Cancer Cell 2003). Our laboratory also demonstrated that specifically at concentrations that are efficacious in vivo, 2ME2 depolymerizes microtubules and blocks the accumulation of HIF-1α in an oxygen and proteasome-independent manner; thus, 2ME2 blocks subsequent transcriptional activity that could initiate angiogenesis (Mabjeesh et al., Cancer Cell 2003).
Initially, our laboratory demonstrated that both taxol and vincristine inhibit HIF-1α levels and HIF-1 transcriptional activity, despite having opposite effects on MT structure (Mabjeesh et al., Cancer Cell 2003). We eventually confirmed that the microtubule-stabilizing and microtubule-destabilizing drugs taxotere, epothilone B, discodermolide, and colchicine also inhibit HIF activity in a dose-dependent manner (Escuin et al., Cancer Res 2005). We verified that the observed effect is downstream of the drugs’ interaction with the MT and of their disruption, and not due to changes in HIF-1α tagging or rates of degradation (Mabjeesh et al., Cancer Cell 2003). When we prevented HIF-1α degradation with the proteasome inhibitor MG-132 in order to observe de novo synthesis of the protein, we found that there were much slower rates of cellular accumulation, hence lower HIF-1α synthesis, with all the microtubule-targeting drug treatments tested (Escuin et al., Cancer Res 2005). HIF-1 transcriptional activity was reduced accordingly.
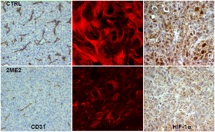
Monastrol use, which does not affect the MTs but does produce mitotic arrest, proved that mitotic arrest alone is not enough to downregulate HIF-1α (Mabjeesh et al., Cancer Cell 2003). However, interphase MT disruption does repress HIF-1α, consequently inhibiting HIF-1 transcriptional activity that includes downregulation of VEGF (Mabjeesh et al., Cancer Cell 2003). Using our drug-resistant cells that were derived from drug-sensitive parental cells under parallel experimental conditions, we were able to directly link the beta-tubulin drug binding of the MT-stabilizing epothilones with HIF-1α protein inhibition (Escuin et al., Cancer Res 2005). The sequence of microtubule disruption, followed by HIF-1α inhibition and impaired nuclear accumulation, was also visualized by laser scanning confocal microscopy. In addition, the Giannakakou laboratory found that the HIF-1alpha protein, but not HIF-1 alpha mRNA, was downregulated in a drug dose-dependent manner (Escuin et al., Cancer Res 2005). Also, in keeping with downregulation of HIF protein levels, HIF-responsive Glut-1 mRNA transcription was affected by drug treatment. However, treatment with the DNA-damaging drug adriamycin at concentrations able to induce p53 and its target gene mdm2 had no effect on HIF-1α levels, showing that changes in HIF-1α protein levels are specific to the microtubule-targeting drugs, and not produced in response to other cellular stressors (Escuin et al., Cancer Res 2005).
Our data support the hypothesis that in vivo, 2ME2 and the tubulin-targeting drugs have a common mechanism that inhibits both tumor cell growth and tumor vascularization by way of disruption of the MT cytoskeleton, ultimately resulting in dividing-cell death as well as downstream effects on the HIF pathway for the non-dividing-cells, leading to inhibition of factors promoting vascularization (Mabjeesh et al., Cancer Cell 2003). All of the MT-targeting drugs tested appear to downregulate HIF-1a protein levels in a dose-dependent manner that solely depends on the act of MT disruption, not specifically stabilization or destabilization, nor any specific tubulin-binding site or drug structure (Escuin et al., Cancer Res 2005). Thus, HIF-1 alpha depends on and can be regulated by changes in cellular MT structure.
Collectively, the Giannakakou laboratory’s results demonstrate that HIF-1α is a target of the microtubule-disrupting drugs, downstream of interphase MT disruption (Escuin et al., Cancer Res 2005). Our data provide the first mechanistic link between targeting of the MT cytoskeleton and inhibition of tumor angiogenesis. This mechanism of action may be an important component of many drugs’ antitumor activities. We believe that the therapeutic targeting of HIF by both existing and more specific drugs should inhibit HIF gene regulation, blocking cancer cell survival: It should reduce angiogenesis and suppress tumor growth while minimally affecting normal, non-hypoxic tissues. The results of our research will have direct implications for the rational design of improved clinical therapies, using new or existing drugs, alone or in combination.
We have secured a grant to investigate in detail the molecular mechanisms that lead from MT disruption to inhibition of both tumor angiogenesis and tumor cell survival. We expect to further elucidate 2ME2’s inhibition of HIF-1α nuclear translocation, plus determine whether motor proteins are involved. We also plan to define the mechanism by which HIF-1α mRNA is controlled and HIF translation is inhibited. Lastly, we will study resistance mechanisms using VHL ubiquitin ligase-mutant cancer cells, examining how those changes affect nuclear translocation and/or translation of HIF-1α. Preliminary microscopy work using molecular beacons to track HIF-1α mRNA and study polyribosome localization in cells appears to indicate that the HIF mRNA is indeed associated with microtubules; while linear sucrose gradient fractionation appears to show that the drugs may affect HIF-1α translation by shifting the polyribosome association profile with HIF mRNA to a translationally-inactive state (Escuin et al., Cancer Res 2005). Future publications will confirm and expand on these exciting discoveries.

Unless indicated otherwise, all content © Paraskevi Giannakakou. All rights reserved.