Confocal Microscopy and Live Cell Imaging

Molecular Basis of Microtubule Drug Resistance
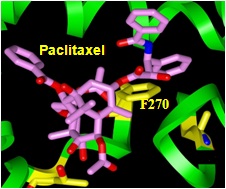
Drugs that bind to either tubulin or microtubules form one of the most effective classes of anticancer agents. This class of drugs is large, and continues to expand. The majority of these compounds are natural products, but they have remarkably diverse chemical structures. Tubulin-targeting agents are divided into two main groups, according to their effects on microtubule polymer mass. The first group is composed of microtubule-destabilizing drugs that bind preferentially to tubulin dimers and inhibit tubulin assembly. These include the Vinca alkaloids, cryptophycins, and colchicine. The second group is composed of microtubule-stabilizing drugs that bind preferentially to the microtubule polymer, enhance tubulin polymerization and include the taxanes (such as Taxol and Taxotere), epothilones, eleutherobins, laulimalide, and discodermolide. Although all these drugs bind to distinct sites on the microtubule or the tubulin dimer, they are all affect microtubule dynamics, block mitosis at the methaphase/anaphase transition, and consequently induce cell death. Since cancer cells are more dynamic and divide at much higher rates than normal cells, the drugs are most toxic to cancer cells (for review look at...)
The clinical success of Taxol, together with the fact that its antitumor spectrum appears to be the broadest of any class of anticancer agents, cause us to argue that microtubules represent the single best anticancer drug target identified to date. However, the emergence of drug resistant tumor cells has limited Taxol's ability to cure disease... read more
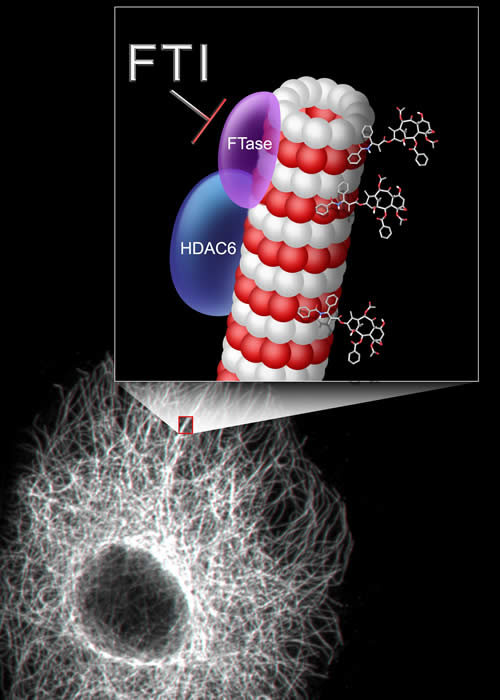
Farnesyltransferase, HDAC6 and Cellular Microtubules
Previous work from our laboratory identified that FTI treatment inhibits the tubulin deacetylase function of HDAC6, thereby enhancing tubulin acetylation and stability and facilitating taxane binding to the stable MT, its preferred substrate [Marcus, A. I., J. Zhou, et al., Marcus, A. I., M. O'Brate A, et al.] . Enhanced taxane binding then results in mitotic arrest and cell death. Interestingly, the FTI/taxane synergy depends on the functional inhibition of both farnesyltransferase (FTase) and HDAC6. Importantly, we also showed that the FTI/Taxane combination is able to reverse taxane resistance, and this effect is also dependent on the presence of a functionally active HDAC6. However, the molecular basis of FTI-induced HDAC6 inhibition is not known. HDAC6 has recently been identified as a deacetylase for tubulin [Hubbert, C., A. Guardiola, et. al., Matsuyama, A., T. Shimazu, et. al., Zhang, Y., N. Li, e.t al.] and the heat shock protein hsp90 [Bali, P., M. Pranpat, et. al., Kovacs, J. J., P. J. Murphy, et. al. , Murphy, P. J., Y. Morishima, et. al.] , and was shown to bind misfolded proteins that are polyubiquitinylated and to transport them to aggresomes via the microtubule-motor dynein [Hook SS, Orian A, et.al.; Kawaguchi Y, Kovacs JJ, et.al.] HDAC6, as opposed to other HDACs, contains two catalytic domains [Grozinger, C. M., C. A. Hassig, et al , Zou, H., Y. Wu, et al.], and one specific pharmacological inhibitor of HDAC6 albeit without strong potency, called tubacin, has been described [Haggarty ,S.J., Koeller K. M., et.al.]. Nevertheless, little is known about cellular factors affecting HDAC6 activity or what other proteins are substrates of HDAC6. ... read more
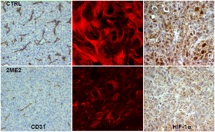
Microtubule-Dependent Regulation of HIF-1α
We have shown that 2-methoxyestradiol (2ME2) inhibits tumor angiogenesis by effectively inhibiting the Hypoxia-Inducible Factor 1α (HIF-1α) levels and transcriptional activity in a variety of human cancer cell lines. HIF-1α inhibition occurs downstream of disruption of the microtubule cytoskeleton by 2ME2. This is verification that HIF-1α is a drugable target and that a functional relationship exists between the antitubulin and antiangiogenic effects of 2ME2. … read more
Cellular Microtubules Keep Tumor Cells Breathing Easy
The transcription factor HIF-1α is essential for a cell's response to low oxygen conditions. Carbonaro et al. demonstrate that production of HIF-1α protein is regulated by dynamic microtubules and that microtubule - targeting drugs shift HIF-1α mRNA into cytoplasmic P-bodies, where its translation is repressed by miRNAs. This biosights episode presents the paper by Carbonaro et al. from the January 10, 2011, issue of The Journal of Cell Biology, and includes an interview with authors Marisa Carbonaro and Paraskevi Giannakakou (Weill Cornell Medical College, New York, NY). Produced by Caitlin Sedwick and Ben Short.
Read the related article online at: http://jcb.rupress.org/content/192/1/83.full